Cohesion at the cellular level: flexible yet stable
Research teams from the Universities of Konstanz and Potsdam are analyzing how proteins work together to enable our cells to both stick and move. The marker protein paxillin is at the centre of their interest.
The tissue in our body can only hold together if the cells adhere not only to each other, but also to extracellular structures, such as collagen fibres of the connective tissue and the skin. How exactly does this work on a cellular level? Which proteins play which role? New data and findings have now been published by the research teams led by cell biologist Christof Hauck (Konstanz, Germany) and chemist Heiko Möller (Potsdam, Germany) in the open access journal "PLOS Biology". The results of their study could contribute to the further development of medical agents that are already being used to treat inflammatory bowel diseases or prevent heart attacks.
Paxillin as a link to the intracellular support system
Specialized membrane proteins – integrins – ensure cohesion in the tissue. They serve as anchoring points for the cells. Every cell has many of these anchoring points, called focal adhesions, which give the cell support like little feet. Integrins have to cooperate with proteins in the cell to be able to also connect to the intracellular support system, the cytoskeleton.
One of these proteins is paxillin. Since paxillin is present in all cells as a link between integrins and the cytoskeleton, it also serves as a marker to visualize the dot-like and line-like anchoring points, i.e. focal adhesions.
Contrary to what the terms cytoskeleton and focal adhesion suggest, these anchoring points are by no means static. During the movement of cells, for example, they are constantly dissolved and reattached elsewhere, such as when connective tissue cells have to close a wound in our skin. The scientists' new data show that paxillin binds directly to the intracellular part of integrin. It clings to the receptor, so to speak.
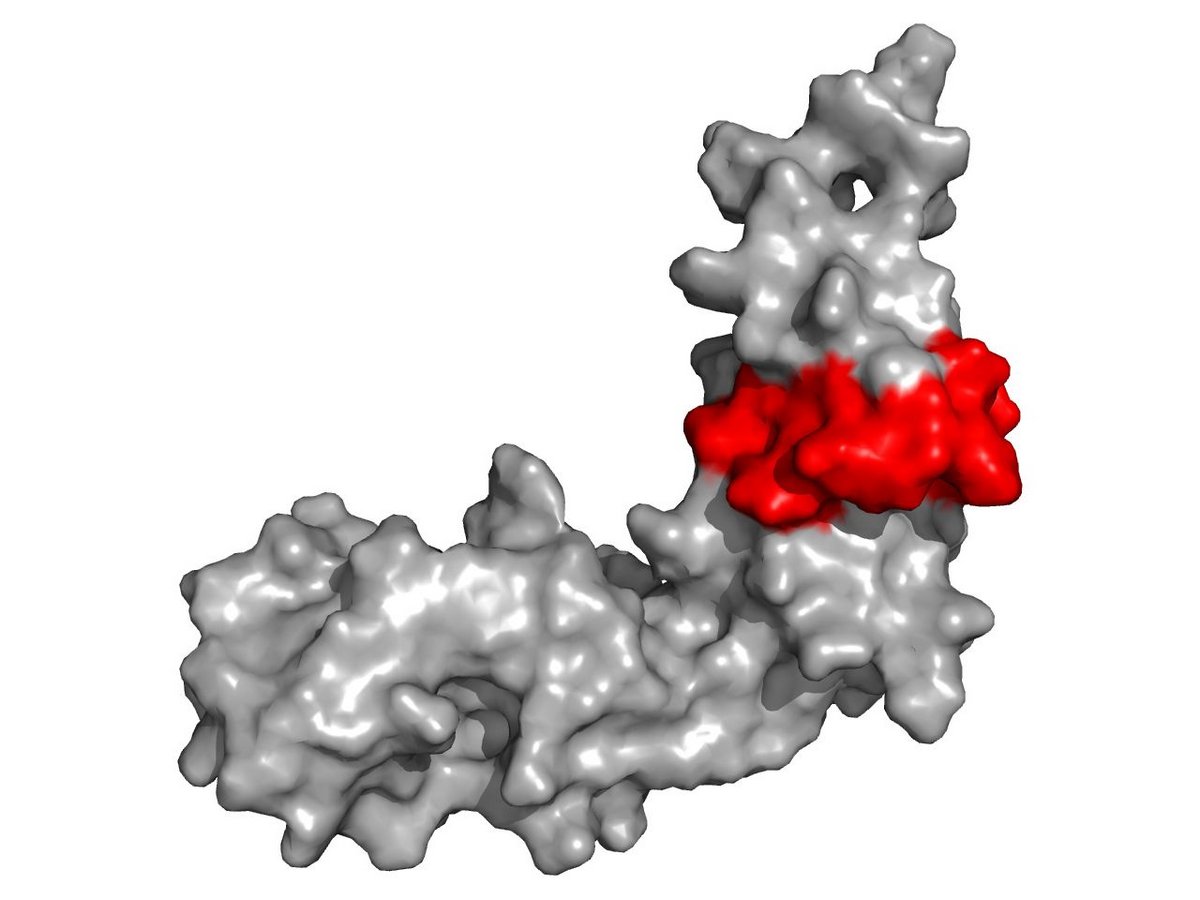
Analysis of the 3D structure reveals important piece of the puzzle
The researchers were able to narrow down the exact interaction site in both paxillin and integrin and determine the previously unknown 3D structure of this part of paxillin.
"We found a crucial piece of the puzzle for understanding the interaction of these two proteins when the exact integrin binding site was delineated in the context of paxillin’s 3D structure: in Paxillin, this part is formed as a movable flap, which is very likely to hold on to the integrin like a clamp, but can also be easily released again," explains chemist Möller. Cell biologist Hauck adds: "In principle, the flexibility of this paxillin segment appears to support the cells' motility as a whole by gripping and releasing the integrin".
Application to medical ingredients
The dynamic protein structures were analyzed using nuclear magnetic resonance spectroscopy (NMR) by Heiko Möller's research group in Potsdam. "This provided the basis for us in Konstanz to produce specific variants of paxillin and integrins, and test in living cells how they affect the formation and composition of focal adhesions. We can now formulate new hypotheses as to how these are formed and remodelled," says Hauck.
In medicine, active substances that manipulate integrins and their ability to adhere are already in use to prevent heart attacks or treat inflammatory bowel diseases. The scientists hope that the results of the study will contribute to the future development of new active substances to specifically target cellular adhesion points.
Key facts:
- Original publication: Baade, T., Michaelis, M., Prestel, A., Paone, C., Klishin, N., Herbinger, M., Scheinost, L., Nedielkov, R., Hauck C.R., Möller H.M. (2024) A flexible loop in the paxillin LIM3 domain mediates its direct binding to integrin β subunits. PLOS Biology; doi: 10.1371/journal.pbio.3002757
- The team led by Professor Christof Hauck, cell biologist in the Department of Biology at the University of Konstanz, is working with colleagues from the University of Potsdam to elucidate the interaction between integrins and the marker protein paxillin.
- A better understanding of the connection between integrins and the cytoskeleton can accelerate the development of drugs to block integrin function, e.g. in connection with thrombus formation, wound healing and inflammation.
- The research was carried out as part of the Collaborative Research Centre (SFB 969) "Chemical and Biological Principles of Cellular Proteostasis" at the University of Konstanz, funded by the German Research Foundation (DFG), and supported by the DFG Research Training Group (RTG 2473) "Bioactive Peptides" at the University of Potsdam.