The gatekeeper of the protein factory
How are proteins sorted in the cell? International research team solves this decade-old puzzle
Researchers solve the more than 25-year-old puzzle of how proteins are sorted in the cell. A protein complex known as NAC (nascent polypeptide-associated complex) serves as a "gatekeeper" in protein synthesis, regulating the transport of proteins within the cell. The molecular mechanism behind this function has now been elucidated by cell and molecular biologists from Konstanz within an international collaborative project.
For the maintenance of our cellular functions, it is essential that proteins are transported to various destinations within the cell – referred to as "cell organelles" in analogy to the organs of our body – while they are still being synthesized. But how is it possible to distinguish between different transport destinations and prevent proteins from reaching the wrong organelles? An international research team has now discovered how this complex process is controlled at the molecular level for an important cellular destination – the transport of nascent proteins to a membrane network of the cell, the endoplasmic reticulum.
In their current publication in the journal Science, the researchers were able to show that a protein complex known among experts as NAC, which was discovered more than 25 years ago, plays a decisive role in this process: Like a gatekeeper, NAC ensures that only proteins with the endoplasmic reticulum as destination are passed on to the protein transporter SRP (signal recognition particle). SRP then mediates the transport of the "cargo" to the specified destination. If, on the other hand, a nascent protein has a destination other than the endoplasmic reticulum, the gatekeeper NAC denies access to the protein transporter SRP.
Protein factory
Using the genetic material as a blueprint, thousands and thousands of new proteins are produced every minute in the cells of our body. This protein production takes place in the ribosomes, the cellular "factories" of our bodies, where individual amino acids – the building blocks of proteins – are assembled into long amino acid chains. The resulting proteins can later take on a wide variety of functions and accordingly have different destinations within the cell. Suitable sorting mechanisms therefore often already ensure during protein production that the proteins reliably reach their respective location within the cell.
Until now, it was known that two protein complexes, the aforementioned NAC and SRP, play an important role in the targeted transport of nascent proteins to the endoplasmic reticulum. SRP is the actual "transport protein" that establishes the contact of the nascent proteins together with the ribosome to the endoplasmic reticulum. It recognizes a specific transport signal that is encoded in the newly synthesized protein. However, there is a problem: SRP also binds non-specifically to ribosomes that have no signal for the endoplasmic reticulum.
"Uncontrolled, SRP would bind to any ribosome close by and then transport it to the endoplasmic reticulum, regardless of whether or not a protein with that destination is currently being produced. This would result in countless misdeliveries that would severely impair the function and viability of the cell", explains Elke Deuerling, one of the senior authors of the current study and Professor of Molecular Microbiology at the University of Konstanz. So the researchers conclude that there is a control instance that prevents exactly that: the gatekeeper NAC.
Tracking down the molecular mechanism
How exactly NAC prevents SRP from binding non-specifically to any ribosome at the molecular level and instead ensures that only the correct ribosomes are transported to the endoplasmic reticulum was previously unclear. The biologists from Konstanz investigated this question in their current study in collaboration with colleagues from ETH Zurich (Switzerland), MRC Laboratory of Molecular Biology (LMB, Cambridge, UK) and the California Institutes of Technology (Caltech, Pasadena, USA).
To do this, they first simulated the processes in the cell by mixing purified ribosomes together with NAC and SRP in the test tube. The mixture was then snap-frozen at below -150°C and the sample examined under an electron microscope – a method known as cryoelectron microscopy. This allowed structural biologists Dr Ahmad Jomaa and Dr Viswanathan Chandrasekaran, co-authors of the study, to reveal how NAC binds to ribosomes before and after cargo transfer to SRP. This was an important cornerstone in elucidating the gatekeeper mechanism, but the transition between the states remained unclear.
"The transition is a highly dynamic process that cannot be visualized by cryoelectron microscopy," explains Dr Martin Gamerdinger, one of the lead authors from the University of Konstanz. To understand this process, he and his team, doctoral researchers Annalena Wallisch and Zeynel Ulusoy, conducted high-resolution biochemical binding studies that revealed in detail the interaction mechanism of NAC on ribosomes depending on the type of protein synthesized.
NAC as a gatekeeper
Using this method and computer-assisted reconstruction of the 3D structures, as well as experiments by Dr Hao-Hsuan Hsieh on the binding strength between the components involved, the researchers succeeded in deciphering how NAC works at the molecular level. Based on their results, they were able to suggest a detailed molecular mechanism for NAC's sorting function.
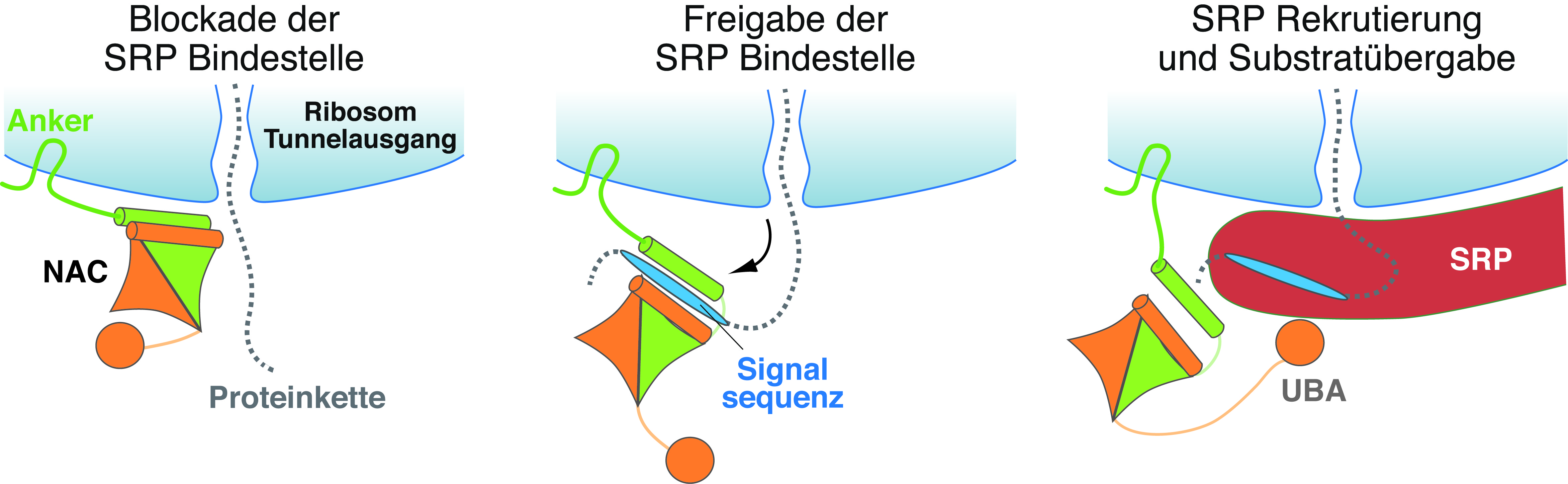
According to this, NAC binds to the ribosome, specifically to the section where the nascent protein leaves the "protein factory". Like a gatekeeper, part of NAC sits protectively in front of this exit, the ribosomal tunnel, and denies SRP access to the ribosome and the nascent protein. Access is only granted when a transport signal sequence for the endoplasmic reticulum – encoded in the nascent protein – leaves the tunnel in the course of the protein synthesis. NAC recognizes this signal and changes its position on the ribosome. This way, the exit of the ribosomal tunnel becomes unblocked and SRP can now dock to the tunnel exit after being actively recruited to the ribosome via a "grabbing arm" of NAC, i.e. the UBA domain. After SRP binding and signal sequence transfer, the ribosome together with the nascent protein is transported to the endoplasmic reticulum.
"Our study reveals the molecular function of NAC as a gatekeeper, granting SRP only access for those nascent proteins whose destination is the endoplasmic reticulum", Professor Elke Deuerling summarizes this fundamental control mechanism. She agrees with her international cooperation partners Professor Nenad Ban (ETH Zurich, Switzerland), Professor Shu-ou Shan (Caltech, USA) and Professor Ramanujan Hegde (MRC-LMB, UK): "Future studies will have to show whether NAC also has other control functions at the ribosomal tunnel."
Key facts:
- Original publication: A. Jomaa, M. Gamerdinger, H.-H. Hsieh, et al. (2022) Mechanism of signal sequence handover from NAC to SRP on ribosomes during ER-protein targeting. Science; DOI: 10.1126/science.abl6459
- Decade-old puzzle solved: International research team from Germany, Switzerland, England and the USA sheds light on molecular control mechanism of sorting of nascent proteins at the ribosomal tunnel.
- NAC (nascent polypeptide-associated complex) acts as a "gatekeeper" at the ribosomal tunnel exit and mediates contact with a suitable transport protein (here SRP: signal recognition particle) only for those nascent proteins whose target organelle is the endoplasmic reticulum.
- Participating institutions: University of Konstanz (Germany), ETH Zurich (Switzerland), MRC Laboratory of Molecular Biology (Cambridge, UK) and California Institute of Technology (Pasadena, USA)
- Funding sources: German Research Foundation (DFG, SFB 969), Swiss National Science Foundation (SNF), Rössler Prize (ETH Zurich), Ernst Jung Prize for Medicine (Jung Foundation) and Otto Naegeli Award (Bonizzi Theler Foundation), National Institutes of Health (NIH) and National Science Foundation (NSF), UK Medical Research Council (MRC), Wellcome Trust, Agouron Institute and Louis-Jeantet Foundation

